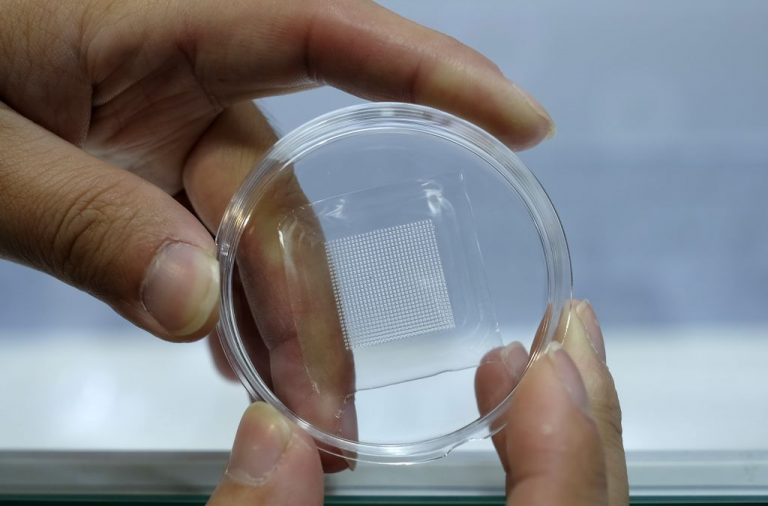
A UK-based health company announced it will begin phase 1 clinical trials of a novel Coronavirus Disease 2019 (COVID-19) vaccine that utilizes a microneedle patch in Switzerland starting in 2022.
Emergex Vaccines Holding Limited made the announcement in a Nov. 15 press release, stating its trial format would be conducted at the Center for Primary Care and Public Health at the University of Lausanne in Switzerland. The trial’s format will involve 13 test subjects and 13 placebo cases in a “double-blind, randomised and comparator-controlled study.”
The trial is set to begin at the beginning of January, 2022.
Emergex touts itself as “a company tackling major global infectious disease threats through the development of 100% synthetic T Cell priming vaccines,” and notes it already has a dengue fever vaccine trial underway in Switzerland where test subjects have already accepted double doses.
Crunchbase says the company has been funded by a slightly-less-than 1 million pound grant from the UK Government’s Innovate UK in 2018 and an $11 million USD Series A funding round from Singapore-based Vickers Venture Partners in January of 2020.
Success
You are now signed up for our newsletter
Success
Check your email to complete sign up
The press release says that the method employed in its microneedle patch vaccine is to attempt to stimulate “naive” CD8+ T-Cells to create Cytotoxic T Lymphocytes (CTLs) specialized against SARS-CoV-2, the virus that causes COVID-19.
By comparison, today’s novel gene therapy adenovirus vector and messenger RNA vaccines involve issuing a genetic instruction to human cells to cause your body to grow the SARS-CoV-2 spike protein for the purpose of eliciting an antibody response that will attempt to stop the real virus.
The company claims the approach is more effective against rapidly mutating viruses, such as the coronavirus family, and has the potential to eliminate the need for perpetual booster injections.
Emergex further states that its vaccine “is raised against antigens that are highly conserved,” which “may provide cross reactive immunity to SARS-CoV-1 infection and all SARS-CoV-2 variants and strains of the virus.”
A promotional page on the Emergex website says their vaccines are “100% synthetic” and contain neither RNA, DNA, or inactivated or live pathogens.
A primer on the British Society for Immunology’s website explains that CD8+ T-Cells/CTLs “are very important for immune defence against intracellular pathogens, including viruses and bacteria, and for tumour surveillance.”
The Society says CTLs kill infected or malignant cells using one of three techniques: the secretion of cytokines, “which have anti-tumour and anti-viral microbial effects,” the manufacture of cytotoxic granules composed of two families of proteins that open a hole in an infected cell for the purpose of cleaving proteins and triggering apoptosis, and “Fas/FasL interactions,” which involve T-Cells binding to infected cells, which is also to trigger apoptosis.
The introduction warns, however, that T-Cell response is not without its potential risks, “In addition to their critical role in immune defense against viruses, intracellular bacteria, and tumours, CD8+ T cells can also contribute to an excessive immune response that leads to immunopathology, or immune-mediated damage.”
While T-Cells function to destroy already infected cells, antibodies generated by conventional vaccines and the novel COVID injections seek out a virus for the purpose of eliminating it before it can infect cells.
Once a virus enters cells and begins to utilize a human cell as a reproductive facility, antibodies become powerless.
The company has a U.S. connection. In September of 2020, Emergex acquired the “laboratories, technology and assets” of Doylestown, Pennsylvania-based ImmProNano (IPN) for an undisclosed amount, forming an American subsidiary dubbed Emergex USA.
The press release noted that, “IPN has completed all pre-clinical testing in animals and ex vivo human samples for Emergex’s recent vaccine programs including Francisella tularensis, Yellow fever, Zika, Dengue, Influenza and COVID-19.”
Just a few days prior to the announcement, San Diego ABC affiliate News 10 published a general segment promoting the use of microneedle patches to deliver COVID vaccination, “They’re using 3D printing to create micro-needle patches. About the size of a fingertip, the patches go on like a Band-Aid. They have hundreds of tiny needles, each coated with a mixture of the COVID-19 vaccine and sugar.”
Stanford researcher, Joe DeSimone, told the outlet, “After you wear it for a couple of hours, that sugar will dissolve into the skin and deliver the vaccine, right where all those immune cells that are targeted.”
“When we get the vaccine in the muscle in our arm, it’s missing a lot of the cells that we’re actually targeting…But what’s interesting is we have 1000-fold more of those cells just underneath the skin, in the dermis.”
The article said DeSimone is working with researchers at the University of North Carolina, a hotbed of Chinese Communist Party and U.S.-joined gain of function research, to create microneedle patches.
Meanwhile, a team of mostly Chinese researchers at Emory University in Atlanta had a paper published in the journal PNAS on Nov. 9 describing the process of creating an “ePatch” based on an electroporation system based on microneedles to inject DNA-based SARS-CoV-2 vaccines into the skin.
Electroporation is described by ThermoFisher as “a physical transfection method that uses an electrical pulse to create temporary pores in cell membranes through which substances like nucleic acids can pass into cells.”
The study says it was able to achieve a unit cost of less than $1 USD and a weight of less than 50 grams by “combining a thumb-operated piezoelectric pulser derived from a common household stove lighter that emits microsecond, bipolar, oscillatory electric pulses and a microneedle electrode array that targets delivery of high electric field strength pulses to the skin’s epidermis.”
Researchers described antibody response generated by the device in mice as “strong,” noting the technique “enabled at least 10-fold dose sparing compared to conventional intramuscular or intradermal injection of the DNA vaccine.”
Emergex is also currently recruiting for a U.S.-based phase 1 clinical trial for its wares, according to the National Libraries of Medicine Clinical Trials website.
In September, researchers at the University of California Riverside announced they are working on genetically engineering lettuce and other plants to synthesize COVID vaccines through their chloroplasts.