A newly released preprint study by researchers from the New York State Department of Health examining data from three large statewide databases has found Coronavirus Disease 2019 (COVID-19) vaccine efficacy (VE) is notably reduced to as little as 12 percent in kids aged 5 to 11 compared to their 12-to-17-year-old counterparts.
In the study submitted to the medRxiv preprint server Feb. 28, researchers examined a period between Nov. 29, 2021 and Jan. 30, 2022, pulling vaccine acceptance data from the Citywide Immunization Registry and the NYS Immunization Information System, which collects “COVID-19 provider vaccination data for residents of New York City and the rest of NYS, respectively.”
The data was combined with test results from the Electronic Clinical Laboratory Reporting System and hospitalization records gathered from the Health Electronic Response Data System in order to assess case counts and hospitalizations.
RELATED READING:
- Pfizer Vaccine Modifies DNA In as Little as 6 Hours: Study
- Brace Yourselves, Pfizer’s Three Dose Omicron Vaccine is Coming
- Kids Perform Propaganda Discriminating Against Unvaccinated Peers at Manhattan School Holiday Show
Children were considered fully vaccinated if they had completed their double injection of Pfizer-BioNTech’s BNT162b2 gene therapy messenger RNA offering at least 14 days prior.
Success
You are now signed up for our newsletter
Success
Check your email to complete sign up
Pfizer’s offering is currently the only product available to kids aged 5 to 11.
“During Omicron variant predominance, VE against infection declined rapidly for NYS children 5-11 years, with low protection by one month following full-vaccination. Among children 12-17, protection declined substantially, albeit more slowly than observed among younger children,” reads the study’s Discussion section.
The provided raw data showed that during the week of Dec. 13 to 19, 2021 that vaccine acceptance in the 5 to 11 bracket had only reached 4.7 percent of the eligible population, but climbed to 23.4 percent by the week of Jan. 24 to 30, 2022.
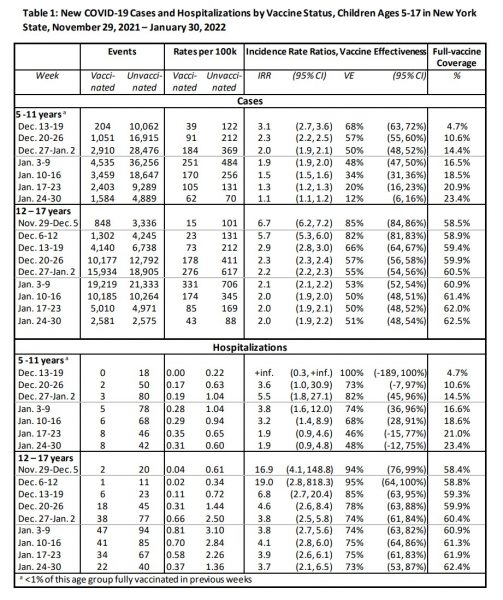
Vaccine acceptance for kids aged 12 to 17 meanwhile had moved only a few percentage points from 59.3 to 62.4 percent over the same period of time.
The study states the raw numbers for fully vaccinated kids amounted to 852,384 in the 12 to 17 bracket and 365,502 in the 5 to 11 bracket.
The Omicron variant of SARS-CoV-2, the virus that causes COVID-19, was originally found in South Africa in late November of 2021, one of the earliest sightings was in four fully vaccinated diplomats from an unknown country who visited Botswana and tested positive as early as Nov. 7.
The study notes that Omicron amounted to 19 percent of all NYS genomic sequencing data as of Dec. 13, rising to 99 percent by Jan. 24.
While vaccine efficacy expectedly collapsed in the face of the Omicron variant for both age brackets, the data nonetheless revealed a sharp contrast.
For the 12 to 17 age bracket, VE began at an estimation of 85 percent during the week of Nov. 29, falling to to 66 percent from 85 percent the week of Dec. 13, and further to 51 percent by Jan. 24
By comparison, in 5-to-11-year-olds, VE started off at 68 percent the week of Dec. 13 and fell to a staggering 12 percent by Jan. 24.
The team theorized that the lower dose of mRNA given in the pediatric version of Pfizer’s injection, two doses of 10 ug compared to two doses of 30 ug, may be the cause of the discrepancy.
Researchers acknowledged the study had some limitations, such as, “Home testing, which is not reported and increased during the analysis period, would impact conclusions if testing practices differed by vaccine status.”
Additionally, data showed that 12.5 percent of the 12 to 17 year old age bracket had accepted a third booster injection, which may have bolstered the segment’s VE figure.
The study also noted, “This analysis compared early vaccinators in the younger age group with later vaccinators in the older age group, who may differ in test-seeking or exposures.”
















