An FDA release of Pfizer’s internal pharmacovigilance reports on vaccine adverse reactions for its novel, gene therapy Coronavirus Disease 2019 (COVID-19) vaccine reveal women who suffered significant side effects outnumbered men by a ratio of more than 3:1, and that the bulk of reactions afflicted people of both genders aged 31 to 50.
The data was released by the Public Health and Medical Professionals for Transparency (PHMPT), a non-profit composed of 80 individuals that are mostly medical professionals.
RELATED ARTICLES:
- Fired Whistleblower in Phase 3 Pfizer COVID Vaccine Clinical Trial Study Location Says Data was Falsified
- Experiment Finds Pfizer Vaccine Desaturates and Clots Red Blood Cells, Contains ‘Garbage’ Crystalline Structures and Fibers Under Microscopy
- Trio of Pfizer Scientists Expose Surveillance and Profit-driven Corporate Culture, Virtues of Natural Immunity on Hidden Camera
55 long years
The PHMPT says on its website that it filed a FOIA request with the FDA on Aug. 23 after Pfizer was granted approval to distribute its injection to youth aged 16+ for “all of the data within Pfizer’s COVID-19 vaccine biological product file.”
During the filings, the organization specifically requested expedited processing, which it notes in its application letter that United States Code allows for.
Success
You are now signed up for our newsletter
Success
Check your email to complete sign up
The FDA denied the PHMPT expedited processing, saying that the application did “not demonstrate[] a compelling need that involves an imminent threat to the life or physical safety of an individual” or “that there exists an urgency to inform the public concerning actual or alleged Federal Government activity,” according to filings.
As a result, the PHMPT lodged a lawsuit on Sept. 16 in the U.S. District Court of Texas, seeking to compel the Agency to fulfill the request.
In mid November, the Department of Justice, which represents the FDA, told the courts it would need 55 years to review and redact information from the 329,000 pages of documents on hand at a rate of 500 pages per month.
Lawyers for the DOJ told the courts the government entity that processes FOIA requests only has ten staff members and is sitting on 400 applications, according to Reuters,
The article said Judge Mark Pittman, who presides over the case, set a conference date for Dec. 14 “to consider the timeline for processing the documents.”
Better than VAERS
Nonetheless, the PHMPT does have a copy of a document prepared by Pfizer’s Worldwide Safety division titled Cumulative Analysis of Post-authorization Adverse Event Reports. The paper contains adverse reaction data collected by the Big Pharma crown jewel through to Feb. 28, 2021, just months after its injection was the first to be granted an Emergency Use Authorization in December of 2020.
The document is dated by the PHMPT as Nov. 17 and is described within by Pfizer as “an integrated analysis of the cumulative post-authorization safety data, including U.S. and foreign post-authorization adverse event reports.”
There are only a few redactions from the DOJ. Specifically, the number of full time staff Pfizer says it had hired and was planning to hire “to help alleviate the large increase of adverse event reports,” and the number of injections it had shipped worldwide by this time.
Pfizer’s internal vaccine adverse reaction database, which the report is based on, contains information gathered from:
- Spontaneous reports to Pfizer
- Reports from health authorities
- Cases published in medical literature
- Those from “Pfizer-sponsored marketing programs” and “non-interventional studies”
- Cases “of serious AEs [adverse reactions]”
- Reports from clinical studies “regardless of causality assessment”
Pfizer adds a disclaimer to their data set, which states both that, “Reports are submitted voluntarily, and the magnitude of underreporting is unknown,” and that “in some reports, clinical information (such as medical history, validation of diagnosis, time from drug use to onset of illness, dose, and use of concomitant drugs) is missing or incomplete, and follow-up information may not be available.”
The company does note, however, that “only those [AEs] having a complete workflow cycle in the safety database” are included in the report.
All the same, the company also reveals that despite “the large numbers of spontaneous adverse event reports received for the product,” that Pfizer still “prioritised the processing of serious cases.”
Hard data
Against a redacted number of doses shipped worldwide as of Feb. 28, 2021, Pfizer revealed that its dataset, composed of the above criterion, contained 42,086 cases with 25,379 being medically confirmed.
More than 27,000 of the total cases were reported from the United States and the United Kingdom.
In a demographic overview, Pfizer revealed a startling figure: 29,914 of the cases were reported in females, while only 9,182 were reported in males. An additional 2,990 were of unknown gender.
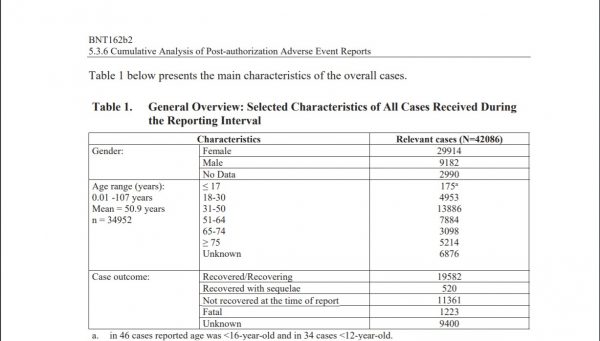
Additionally, broken down by age, while 18-30 year olds made up 4,953 cases and 51-64 year olds made up 7,884 cases, 31-50 year olds were by far the largest segment, registering 13,886 adverse reactions.
Additionally, Pfizer revealed 2.9 percent of all cases, 1,223 in total, carried a fatal outcome.
FURTHER READING:
- Twitter Labels Obituary of 37-Year-Old Seattle Mother Who Died From COVID Vaccine Blood Clots ‘Misleading’
- 45-Year-Old Baltimore Woman Dies After Being Required by Employer to Take COVID-19 Vaccine
- UK Model Takes AstraZeneca Injection, Suffers Brain Hemorrhage, Coma; Dies 16 Days Later
A further 11,361 cases were categorized with the outcome “not recovered at the time of report,” and 9,400 were listed as “unknown.”
Additionally, while the most commonly reported adverse reactions were side effects such as headache, nausea, and fatigue, the document showed the occurrence of 1,927 reports of COVID-19 infection, a rate of 4.5 percent.
Anaphylaxis
In a table titled Important Potential Risk, Pfizer noted 1,833 “potentially relevant cases” of anaphylaxis, which it says were “individually reviewed and assessed according to Brighton Collaboration (BC) definition.”
BC levels are divided into five ranks. The paper says that BC1 “indicates a case with the highest level of diagnostic certainty,” while “diagnostic certainty is lowest” for BC3.
Level 4 is regarded as a “reported event of anaphylaxis with insufficient evidence to meet the case definition” and Level 5 was determined to not be anaphylaxis.
Data reveals that of the 1,833 cases, 601 were classified as BC1 or BC2.
In a demographic breakdown of 1,002 cases meeting BC1 through BC4, the majority of all anaphylaxis occurred in the U.S. and the UK.
Broken down by gender, the data once again showed that females were hit significantly harder, this time at a rate of more than 8 to 1: 876 females and 106 males were in the cohort, with 20 cases afflicting those with “unknown” gender.
The mean age was 42.5 years, and nine deaths were registered. In a footnote associated with the death stats, Pfizer said, “There were 4 individuals in the anaphylaxis evaluation who died on the same day they were vaccinated.”
“Although these patients experienced adverse events (9) that are potential symptoms of anaphylaxis, they all had serious underlying medical conditions, and one individual appeared to also have COVID-19 pneumonia, that likely contributed to their deaths.”
Vaccine enhanced diseases
In a second Important Potential Risk table, Pfizer discussed Vaccine Associated Enhanced Disease (VAED) and Vaccine Associated Enhanced Respiratory Disease (VAERD), but had little data to report, stating, “No post-authorized AE reports have been identified as cases of VAED/VAERD, therefore, there is no observed data at this time.”
Nonetheless, when Pfizer parsed its data for cases “indicating a lack of effect of the vaccine” or “potentially indicative of severe or atypical COVID-19,” it uncovered 138 cases.
71 cases were noted to be “medically significant,” with 8 marked as “serious for Disability.”
38 were flagged as resulting in a fatal outcome.
By gender, the set was composed of 73 females, 57 males, and 8 unknowns.
In addition to the 138 cases, 317 “potentially relevant events were retrieved.” The most frequently reported was “drug ineffective” at a count of 135.
‘Spontaneous abortion’
Pfizer’s data also found 413 cases in a third Important Potential Risk table regarding “use in pregnancy and lactation.” Of the cases, 84 were flagged as serious and 329 as non-serious.
The majority, 205, occurred in the United States. Next was 64 in the UK. Of the 413 cases, 270 involved pregnancies with 4 cases occurring in the fetus/baby.
Among the 270 pregnancies, Pfizer admitted that 23 resulted in “spontaneous abortion,” with two additional cases each of “premature birth with neonatal death” and “spontaneous abortion with intrauterine death,” and one further case of “spontaneous abortion with neonatal death.”
Additionally, Pfizer reported 133 cases of adverse reactions in breastfeeding babies, which included 116 instances where the baby was exposed to the vaccine via breast milk, but without incurring adverse reaction.
















